TricoVEG ™
| Commercial name | TricoVEG ™ |
| Apllication | Hair Care |
| Function | Anti-Hair Loss |
| Use level | 1-5% |
Suggested Applications
- Anti-hair loss leave-on treatment
- Shampoo
- Conditioner
Androgenetic alopecia is the most common type of hair loss, resulting from the susceptibility of hair follicles to androgenetic miniaturization. Recent studies have highlighted the involvement of various inflammatory activators in the etiology of androgenetic alopecia, contributing to the involution of the pilosebaceous unit.
While not a disease in itself, androgenetic alopecia is often experienced as a profound distress, leading to negative psychological and social repercussions.
It is an androgen-dependent and genetically acquired disorder caused by excessive 5-alpha-reductase (5AR) enzyme activity in the hair follicle. Using NADPH as a cofactor, 5AR converts testosterone into the androgen hormone dihydrotestosterone (DHT), which acts on DNA, inhibiting protein synthesis in hair germ cells, leading to bulb miniaturization and irreversible hair loss.
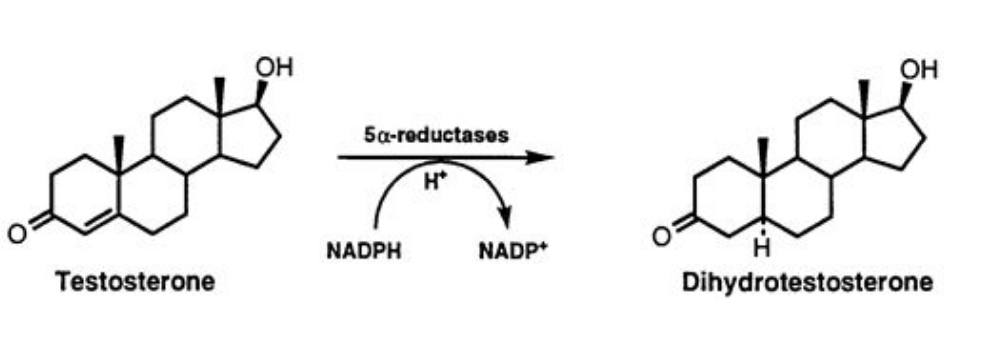
As a result, hair becomes increasingly shorter and thinner, eventually unable to adequately cover the scalp. This occurs because the anagen (growth) phase progressively reduces in favor of the catagen (involution) and telogen (rest) phases.
Hence, the importance of topically applying active ingredients capable of inhibiting 5-alpha-reductase activity to counteract hair loss and induce cell proliferation in hair follicles.
Efficacy Assessment
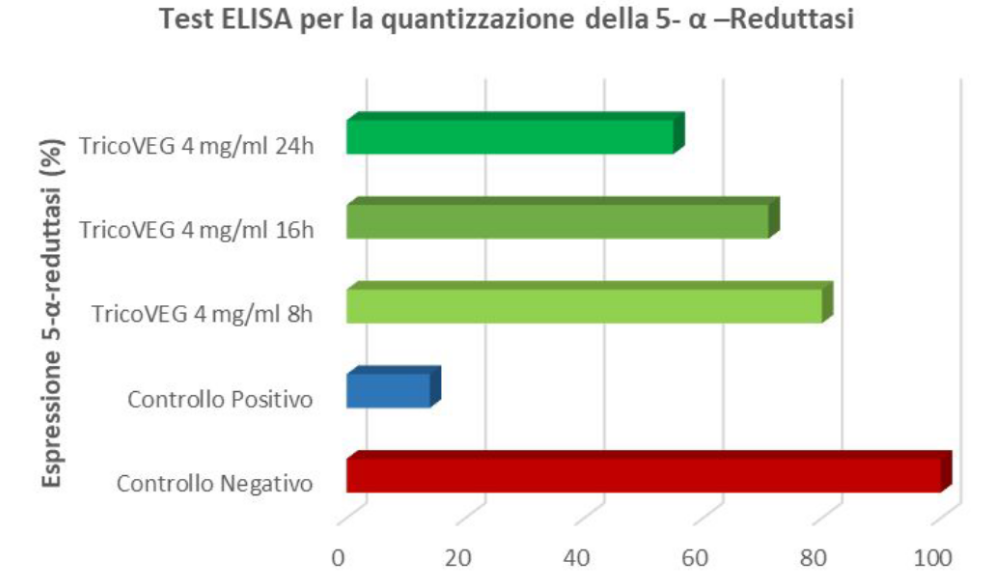
DETERMINATION OF 5-ALPHA-REDUCTASE INHIBITION – ELISA Test for 5-alp
Immunological techniques are based on the specificity of antibodies to recognize and bind to the target protein, offering several advantages over commonly used protein analysis techniques.
ELISA protocols have been developed, using an indirect approach, for the identification of 5-alpha-reductase in the culture medium of treated HHDPCs (human hair dermal papilla cells). These tests help assess the efficacy of the active ingredients in inhibiting the 5-alpha-reductase enzyme and promoting hair follicle cell proliferation.
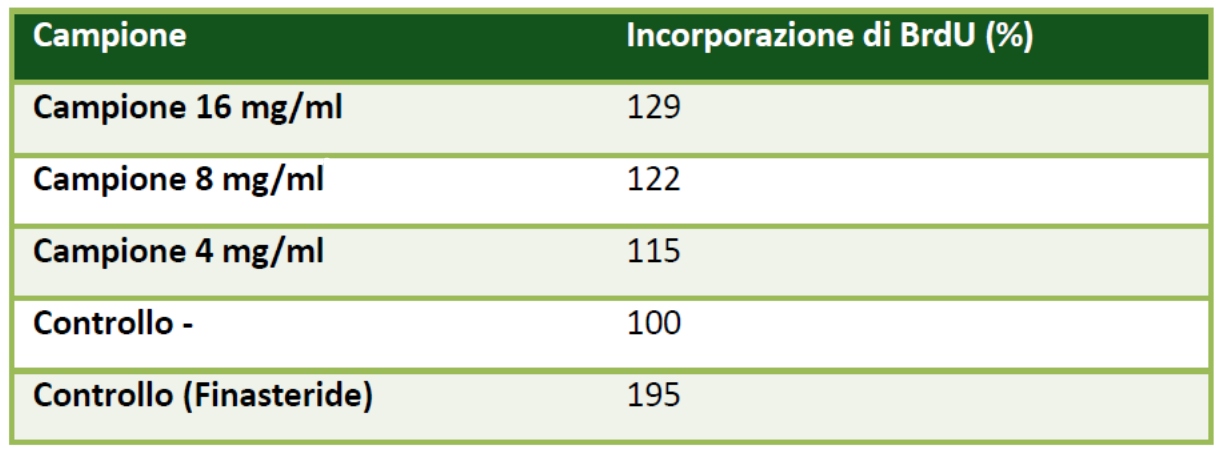
Based on the results of cell viability, a sample concentration of 4 mg/ml was used for the present test. A control sample was prepared by replacing the sample volume with an equal volume of culture medium. Finasteride was used as the positive control. The results are shown in Figure 1.
DETERMINATION OF DERMAL PAPILLA CELL PROLIFERATION THROUGH BROMODEOXYURIDINE INCORPORATION ASSAY
The effect of TricoVEG on cell proliferation was examined using a colorimetric immunoenzymatic assay based on bromodeoxyuridine (BrdU) incorporation using a BrdU kit (Roche, Mannheim, Germany) following the manufacturer’s protocol. Briefly, HHDPC cells (5,000 cells/well) were seeded in 96-well plates.
After 24 hours, the cells were incubated for 48 hours with different concentrations of the sample (4, 8, 16 mg/ml), and finasteride (0.037 μg/ml) was used as the positive control for 5-alpha-reductase enzyme inhibition and, consequently, cell proliferation inducer. Then, 20 μl of BrdU solution was added, and the cells were further incubated for 4 hours.
During this incubation, the pyrimidine analog BrdU was incorporated into the DNA of proliferating cells in place of thymidine. Subsequently, the cells were fixed and denatured with the FixDenat solution from the kit for 30 minutes at 25 °C. This denaturation of DNA is necessary to improve the accessibility of the incorporated BrdU and facilitate its detection by the antibody. The incorporation index in the DNA of dividing cells is directly proportional to the cell proliferative effect. The samples were incubated for 90 minutes with a peroxidase-labeled anti-BrdU antibody (anti-BrdU-POD), which binds to the BrdU incorporated into the newly synthesized cellular DNA.
After washing away the unbound anti-BrdU-POD, the colorimetric reaction was induced for 3 to 5 minutes with the substrate solution and blocked by adding 25 μl of 1 M sulfuric acid. The optical density of the samples was measured using a plate reader at a wavelength of 450 nm.
The cells treated with TricoVEG at the tested concentration and time points showed good proliferative activity of the examined HHDPC cell line.
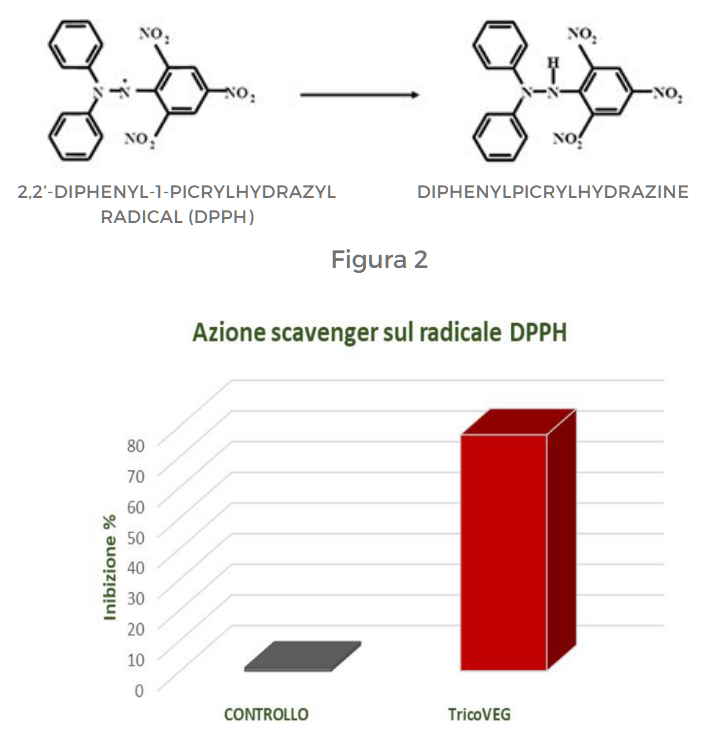
EVALUATION OF ANTIOXIDANT ACTIVITY: SCAVENGING ACTION ON DPPH RADICAL
DPPH (2,2′-diphenyl-1-picrylhydrazyl) is an organic free radical with a maximum absorption peak at 515-528 nm, allowing the assessment of antioxidant activity of different compounds through a spectrophotometric measurement. The reaction involves the reduction of DPPH radical by antioxidant molecules, resulting in the formation of a yellow compound, diphenylpicrylhydrazine (Figure 2).
The extent of the reduction reaction depends on the ability of molecules to act as electron donors. To evaluate the antioxidant properties of the tested product, the scavenging activity against DPPH radicals was determined by adding 0.1 ml of the sample to 9.9 ml of a DPPH solution (200 µM) prepared in ethanol. The absorbance was measured after 15 minutes at 517 nm. The data were expressed as inhibition percentage and reported in Figure 3.
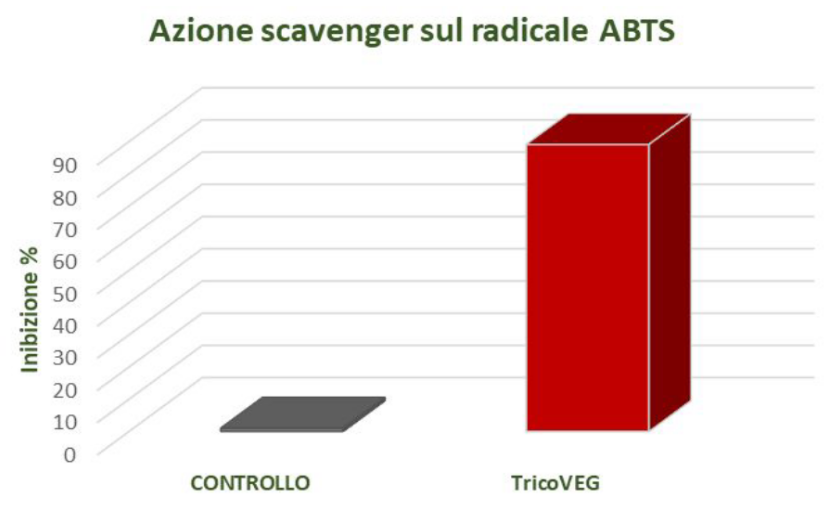
DETERMINATION OF ANTIOXIDANT ACTIVITY: SCAVENGING ACTION ON THE ABTS RADICAL
ABTS (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) is a hydrophilic radical with a maximum absorption around 734 nm. In this case as well, the reaction depends on the ability of the antioxidant to donate hydrogen atoms. The method is based on the ability of antioxidants to act on the ABTS radical cation, a blue-green chromophore with a characteristic absorption at 734 nm. ABTS • + was generated by reacting an aqueous solution of ABTS (7 mM) with potassium persulfate 2.45 mM (K2S2O8).
The resulting mixture was left in the dark at room temperature and, after 16 hours, it was diluted with distilled water to an absorbance of 0.999 ± 0.030 at 734 nm. To evaluate the antioxidant properties of the samples, 0.1 ml of the sample was added to 1 ml of the final ABTS solution and 0.9 ml of distilled water. A control was prepared under the same reaction conditions but using distilled water instead of the sample. After 6 minutes, the absorbance was measured at 734 nm.
The data obtained are expressed as a percentage of radical inhibition and are reported in Figure 4.
The data obtained from the conducted studies have highlighted the ability of TricoVEG to inhibit the ABTS radical.
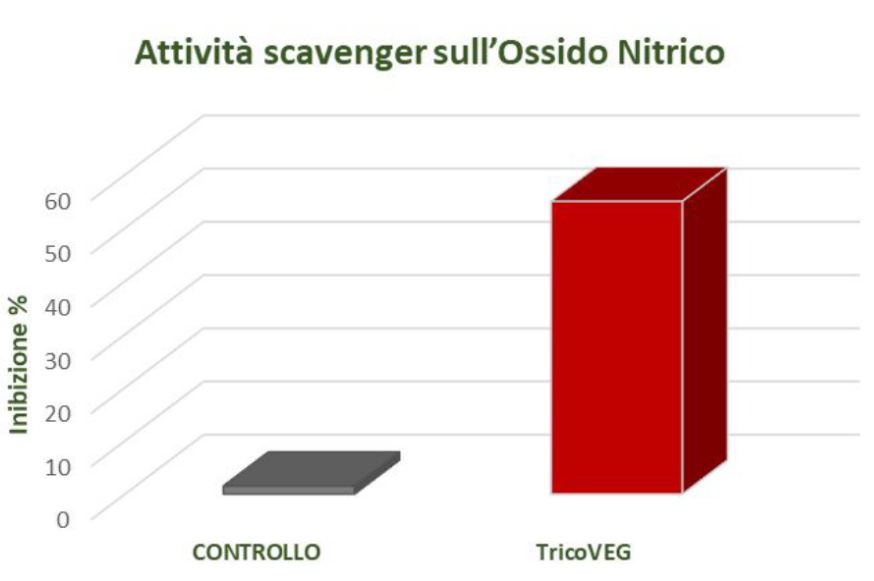
SCAVENGER ACTIVITY ON NITRIC OXIDE – ANTI-INFLAMMATORY ACTION
In vitro determination of scavenger activity on nitric oxide can be a useful indicator of anti-inflammatory activity. Nitric oxide (NO) is a short-lived free radical and an intercellular messenger produced by various mammalian cells, such as macrophages, neutrophils, platelets, fibroblasts, endothelial cells, neurons, and smooth muscle cells.
NO mediates a variety of biological events ranging from vasodilation, neurotransmission, Inhibition of platelet adhesion and aggregation, but it is also involved in a wide range ofinflammatory conditions.
The scavenger action against nitric oxide was determined using the Griess method.
The reaction is conducted using the Griess reagent, which is a mixture of sulfanilamide and 1-naphthylethylenediamine in an acidic environment. Under these conditions, nitrite tends to form nitrous acid, which reacts with sulfanilamide to produce a diazonium salt (diazotization reaction). The diazonium salt then undergoes a coupling reaction with 1-naphthylethylenediamine, resulting in a purple azo dye with a maximum absorption peak at a wavelength of 540 nm.
The scavenger activity against nitric oxide was expressed as a percentage, and the results are shown in Figure 5.